Background: The oral PI3Kδ inhibitor linperlisib has proven to be a significant therapeutic option for patients with relapsed or refractory follicular lymphoma (FL) in a phase II study. As a result, linperlisib was approved in China for the management of adult patients with relapsed or refractory FL who had undergone at least two prior systemic treatments. In addition to linperlisib, several PI3K inhibitors were approved by Food and Drug Administration for relapsed or refractory FL. Despite their confirmed efficacy, these drugs encountered substantial tolerability issues, leading to the rescission from indications for FL. In response to these challenges, we undertook a post-hoc analysis of the phase II linperlisib study to evaluate its safety profile in treating FL, as well as assessing the impact of dose adjustments on prognosis of patients.
Methods: In the single-arm, open-label, multicenter phase II study, eligible patients (adults with histologically confirmed relapsed or refractory FL showing disease progression post at least two prior systemic therapies) were administered daily doses of 80mg linperlisib until disease progression or the emergence of unacceptable toxicity. The primary endpoint was the objective response rate (ORR). For this post-hoc analysis, patients were stratified into three groups: the dose-adjusted group (patients who had dose adjusted or interrupted due to adverse events [AEs]); the treatment-terminated group (patients who ceased treatment for a variety of reasons, which included AEs, withdrawal of informed consent, and the investigator's judgment); and the dose-unadjusted group (patients who experienced neither dose adjustment nor interruption).
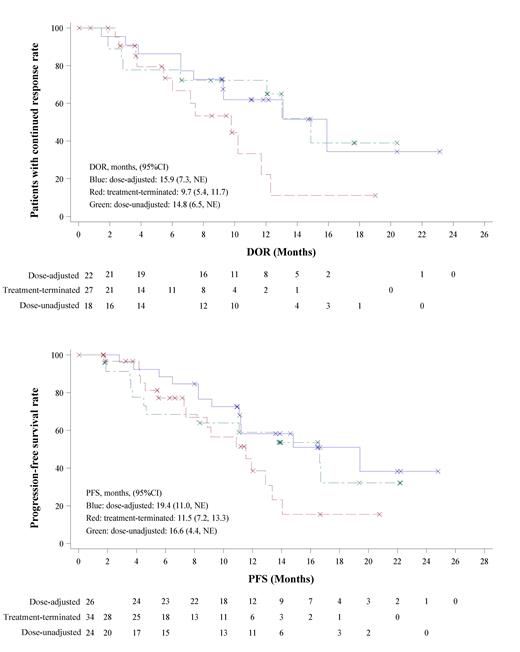
Results: Of 84 patients in the study, dose adjustments or interruptions due to AEs were required for 26 patients, while 34 patients terminated their treatment (15 due to AEs, 6 as per the investigator's discretion, and 14 due to withdrawal of informed consent). A remaining subset of 24 patients experienced no dose modifications. Notably, the dose-adjusted and treatment-terminated groups contained more patients aged 60 and above (19.2% and 29.4% respectively), compared to only 4.2% in the dose-unadjusted group. Other baseline characteristics appeared comparable across all three groups. Among 26 patients in the dose-adjusted group, 25 had dose adjustments due to AEs, and 11 experienced dose interruptions. Infectious pneumonia and interstitial pneumonia were reported in 20.2% (17 cases) and 4.8% (4 cases) of patients, and represent the primary AEs causing treatment termination (9 cases and 4 cases, respectively). Thirteen patients experienced diarrhea during the study, with six requiring dose adjustments, and one patient ceasing treatment due to autoimmune colitis combined with a fungal infection. Rashes, which had an incidence of 11.9% (10 cases), necessitated dose interruptions in four patients but did not require any dose reductions. The ORR was comparable among the three groups (84.6% in dose-adjusted group, 79.4% in treatment-terminated group, and 75.0% in dose-unadjusted group). However, the dose-adjusted and dose-unadjusted groups demonstrated superior duration of response (median DOR, 15.9 months in dose-adjusted group, 9.7 months in treatment-terminated group, and 14.8 months in dose-unadjusted group) and progression-free survival (median PFS, 19.4 months in dose-adjusted group, 11.5 months in treatment-terminated group, and 16.6 months in dose-unadjusted group) than the treatment-terminated group (Figure 1). The median overall survival was not reached in three groups.
Conclusion: Our post-hoc analysis reveals that elderly patients (≥60 years old) have reduced tolerance to linperlisib, necessitating more frequent dose adjustments due to AEs. Notably, treatment discontinuation results in inferior DOR and PFS, while dose adjustments or suspensions yield comparable DOR and PFS to full-dose treatment, suggesting that tolerability-guided dose modification effectively reduces AEs without sacrificing therapeutic efficacy.
Disclosures
Fu:Takeda Pharmaceutical Company Limited.: Research Funding; Shanghai Changzheng Hospital: Other: WJF is a former staff of Shanghai Changzheng Hospital and now is a staff of Shanghai Fourth People's Hospital affiliated to Tongji University. .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal